CENTRIFUGATION
CONTENT:
Rotors,
types of rotor, types of centrifugation, sedimentation principle and
mathematical relationship and application.
A centrifuge
is a device for separating particles from a solution according to their size,
shape, density, viscosity of, and rotor speed. In biology, the particles are
usually cells, subcellular organelles, viruses, large molecules such as
proteins, and nucleic acids. To simplify mathematical terminology, we will
refer to all biological material as spherical particles. There are many ways to
classify centrifugation.
The single
most important advance in the use of centrifugal force to separate biologically
important substances was the combination of mechanics, optics, and mathematics
by T. Svedberg and J.W. Williams in the 1920s. They initiated the mathematics
and advanced the instrumentation. Nowadays, any technique employing the
quantitative application of centrifugal force is known as ultra centrifugation.
Rotors
Rotors for a
centrifuge are either fixed angles, swinging buckets, continuous flow, or
zonal, depending upon whether the sample is held at a given angle to the
rotation plane, allowed to swing out on a pivot and into the plane of rotation,
designed with inlet and outlet ports for separation of large volumes, or a
combination of these. Fixed angles generally work faster; substances
precipitate faster in a given rotational environment, or they have an increased
relative centrifugal force for a given rotor speed and radius. These rotors are
the workhorse elements of a cell laboratory, and the most common is a rotor
holding 8 centrifuge tubes at an angle of 34°C from the vertical. Swinging
bucket rotors (horizontal rotors) have the advantage that there is usually a
clean meniscus of minimum area. In a fixed-angle rotor, the materials are
forced against the side of the centrifuge tube, and then slide down the wall of
the tube. This action is the primary reason for their apparent faster
separation, but also leads to abrasion of the particles along the wall of the
centrifuge tube. For a swinging bucket, the materials must travel down the
entire length of the centrifuge tube and always through the media within the
tube. Since the media is usually a viscous substance, the swinging bucket
appears to have a lower relative centrifugal force, and it takes longer to
precipitate anything contained within. If, however, the point of centrifugation
is to separate molecules or organelles on the basis of their movements through
a viscous field, then the swinging bucket is the rotor of choice. Most common
clinical centrifuges have swinging buckets. Cell biologists employ zonal rotors
for the large-scale separation of particles on density gradients. The rotors
are brought up to about 3000 rpm while empty, and the density media and tissues
are added through specialized ports.
Rotor Tubes
In using
either a fixed-angle or swinging-bucket rotor, it is necessary to contain the
sample in some type of holder. Continuous and zonal rotors are designed to be
used without external tubes. For biological work the tubes are divided into
functional groups, made of regular glass, Corex glass, nitrocellulose, or
polyallomer. Regular glass centrifuge tubes can be used at speeds below 3000
rpm, that is, in a standard clinical centrifuge. Above this speed, the xg forces
will shatter the glass. For work in the higher speed ranges, centrifuge tubes
are made of plastic or nitrocellulose. Preparative centrifuge tubes are made of
polypropylene and can withstand speeds up to 20,000 rpm.
Analytical/Preparative
Centrifugation
The 2 most common types of centrifugation are analytical and preparative; the distinction is between the 2 is based on the purpose of centrifugation. Analytical centrifugation involves measuring the physical properties of the sedimenting particles, such as sedimentation coefficient or molecular weight. Optimal methods are used in analytical ultracentrifugation. Molecules are observed by optical system during centrifugation, to allow observation of macromolecules in solution as they move in the gravitational field. The samples are centrifuged in cells with windows that lie parallel to the plane of rotation of the rotor head. As the rotor turns, the images of the cell (proteins) are projected by an optical system onto film or a computer. The concentration of the solution at various points in the cell is determined by absorption of a light of the appropriate wavelength. This can be accomplished either by measuring the degree of blackening of a photographic film or by the deflection of the recorder of the scanning system or fed into a computer. The other type of centrifugation is called preparative and the objective is to isolate specific particles that can be reused. There are many type of preparative centrifugation such as rate zonal, differential, and isopycnic centrifugation.
Ultracentrifugation/Low-Speed
Centrifugation
Another system of classification is the rate or speed at which the centrifuge is turning. Ultracentrifugation is carried out at speed faster than 20,000 rpm. Super speed ultracentrifugation is at speeds between 10,000 and 20,000 rpm. Low-speed centrifugation is at speeds below 10,000 rpm.
Moving boundary/Zone Centrifugation
A third method of defining centrifugation is by the way the samples are applied to the centrifuge tube. In moving boundary (differential) centrifugation, the entire tube is filled with sample and centrifuged. Through centrifugation, one obtains a separation of 2 particles, but any particle in the mixture may end up in the supernatant or the pellet, or it may be distributed in both fractions, depending upon its size, shape, density, and conditions of centrifugation. The pellet is a mixture of all of the sedimented components, and is contaminated with whatever unsedimented particles were in the bottom of the tube initially. The only component that is purified is the slowest-sedimenting one, but its yield is often very low. The 2 fractions are recovered by decanting the supernatant solution from the pellet. The supernatant can be recentrifuged at a higher speed to obtain further purification, with the formation of a new pellet and supernatant.
In rate
zonal centrifugation, the sample is applied in a thin zone at the top of the
centrifuge tube on a density gradient. Under centrifugal force, the particles
will begin sedimenting through the gradient in separate zones, according to
their size, shape, and density. The run must be terminated before any of the
separated particles reach the bottom of the tube.
In isopycnic
technique, the density gradient column encompasses the whole range of densities
of the sample particles. The sample is uniformly mixed with the gradient
material. Each particle will sediment only to the position in the centrifuge
tube at which the gradient density is equal to its own density, and it will
remain there. The isopycnic technique, therefore, separates particles into zone
solely on the basis of their density differences, independent of time. In many
density gradient experiments, particles of both the rate zonal and isopycnic
principles may enter into the final separations. For example, the gradient may
be of such a density range that one component sediments to its density in the
tube and remains there, while another component sediments to the bottom of the
tube. The self-generating gradient technique often requires long hours of
centrifugation. Isopycnically banding DNA, for example, takes 36 to 48 hours in
a self-generating cesium chloride gradient. It is important to note that the
run time cannot be shortened by increasing the rotor speed; this only results
in changing the position of the zones in the tube, since the gradient material
will redistribute farther down the tube under greater centrifugal force.
Basic Theory
of Sedimentation
Molecules
separate according to their size, shape, density, viscosity, and centrifugal
force. The simplest case is a spherical molecule. If the liquid has the density
of do and the molecule has a density of d, and if d > do, then the protein
will sediment. In gravitational field, the motor force (Pg) equals the
acceleration of gravity (g) multiplied by the difference between the mass of
the molecule and the mass of a corresponding volume of medium.
Equation
1. Pg = (m – m0)g
Equation 2.
Pg = 4/3 (3.14) r3 dg –4/3 (3.14) r3 do g
Equation 3.
Pg = (4/3) r3 (3.14) (d – d0)g
Where,
Pg = force
due to gravity,
g =
acceleration of gravity, do = density of liquid (or gradient)
d = density
of molecule, m = mass of the molecule,
m0 = mass of equal volume of medium.
In a
centrifugal field, the gravitational acceleration (g) is replaced by the
centrifugal force.
APPLICATIONS OF CENTRIFUGATION TECHNIQUE
- separation of different cell organelles.
- separation of bio-molecules in given sample solution.
- to study the density and molecular mass of closely related bio-molecules.
- separation of enzyme from plant extract.
- electrophoresis, chromatography etc are chiefly dependent on centrifugation technique for "pure" sample.
 |
| DIFFERENTIAL CENTRIFUGATION |
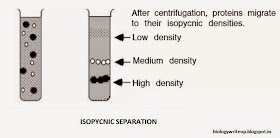 |
| ISOPYCNIC SEPARATION |

I need to reference this. Who posted this please/
ReplyDelete