NERVE SIGNALLING
CELLS OF THE NERVOUS SYSTEM
Cells of the nervous system can be
divided into:
- Neurons or nerve cells: The cells of the nervous system which are capable of electrical signaling over long distances.
- Neuroglia or glia: Supporting cells; they are not capable of electrical signaling but have essential functions in the brain. Eg. Schwann cells which synthesize myelin.
GENERATION AND
PROPAGATION OF ACTION POTENTIAL
- Resting membrane potential: The electrical potential across the plasma membrane of a neuron in the absence of a stimulus. Value ranges from -40 to -90mV.
- Action potential: A series of sudden changes in the electrical potential across the plasma membrane of the neuron, produced in response to a stimulus. Peak value can be as high as +50mV.
- Depolarization: The process by which the membrane potential across the neuronal plasma membrane becomes more positive than the resting potential.
- Repolarization: The process by which the membrane potential across the neuronal membrane is restored to the original resting membrane potential.
- Threshold potential: The level of the membrane potential at which an action potential occurs.
- Refractory period: The interval during which a Na+ channel (which opened in response to an action potential) is inactivated and unresponsive to subsequent action potentials.
Electrical potentials generated
across the membranes of neurons due to -
- Differences in concentrations of specific ions across the cell membrane.
- Activity of active transporters which transport ions against their concentration gradient.
- Selective permeability of the membrane.
- Due to presence of ion channels which allow movement of specific ions along their concentration gradient.
Equilibrium potential is the
electrical potential generated across the membrane at electrochemical
equilibrium. The magnitude is given by the Nernst equation -
 |
| EQUATION FOR EQUILIBRIUM POTENTIAL (NERNST EQUATION) |
where,
- EX: Equilibrium potential for any ion X
- R : Gas constant
- T : Absolute temperature (in Kelvin)
- z : Valence of the permeant ion
- F : Faraday constant (the amount of electrical charge contained in one mole of a univalent ion) (96,000 coulombs/(mol .V).
-
[X]1 , [X]2 : Concentrations of ion X on each side of the membrane.
Equilibrium potential is conventionally
defined in terms of the potential difference between the reference compartment
and the other side
- In biological systems, the outside of the cell is the conventional reference point (defined as zero potential)
The inside-negative resting
membrane potential of the neuron is due to the following factors:
- The Na+/K+ pump generates a high concentration of K+ and a low concentration of Na+ in the cytosol, relative to the extracellular environment.
- The K+ concentration gradient causes outward movement of K+ through non gated K+ channels.
The cycle of depolarization,
hyperpolarization and repolarization constitutes an action potential.
Lasts for 1-2ms and can occur
hundreds of time per second in a neuron. |
| SCHEME OF ACTION POTENTIAL |
Action potentials are generated by the sequential opening and closing of voltage-gated Na+ and K+ channels.
1. Depolarization:
- Small depolarization causes the voltage-gated Na+ channels to open, causing movement of Na+ ions into the cell.
- The excess positive charges on the cytoplasmic phase and the excess negative charges on the exoplasmic face diffuse from the initial site of depolarization.
- This causes the depolarization of adjacent segments of the plasma membrane and opening of more voltage-gated Na+ channels resulting in greater depolarization.
- Due to the increased permeability to Na+, the membrane potential increases, approaching ENA(equilibrium potential for a membrane permeable only to Na+).
- Further net inward movement of Na+ ceases as the membrane potential approaches ENA, as the Na+ concentration gradient is offset by the inside-positive membrane potential.
- After the initial influx of Na+ in response to depolarization, the Na+ channels are inactivated, preventing further Na+ influx.
- The channel remains inactivated for as long as the membrane is depolarized.
- This period, during which the Na+ channel is inactivated and cannot be reopened, is called the refractory period.
- After repolarization, the channel returns to the closed state, to be opened again by depolarization.
- Repolarization of the membrane occurs during refractory period.
- Opening of voltage-gated K+ channels due to depolarization results in efflux of K+ ions.
- The opening of the voltage-gated K+ channels is slightly delayed after the initial depolarization; they open at the height of the action potential. Hence also known as delayed K+ channels.
- Removal of excess positive charges restores the inside-negative resting membrane potential.
- The K+ channels remain open throughout the depolarization phase and close only when the resting membrane potential is restored
- Eventually all the voltage-gated K+ and Na+ channels return to their closed resting state.
- Only the non-gated K+ channels remain open.
 |
| SCHEME OF REPOLARIZATION |
Depolarization of the plasma membrane due to opening of voltage-gated
Na+ channels
Propagation of action potentials
1. The action potential is propagated
without diminution
- Passive spread of the depolarization causes depolarization of an adjacent segment of the membrane.
- This results in the opening of Na+ channels, resulting in an influx of Na+ ions which increases the extent of depolarization.
- Results in the opening of many more Na+ channels, generation of an action potential.
- The K+ channels also open in response to the depolarization, restoring the membrane to the resting potential.
- The action potential thus spreads away from its initial site, without diminution.
- The Na+ channels are inactivated during the refractory period.
- These inactivated channels cannot respond to depolarization by opening.
- This ensures that the action potential is propagated in only one direction, from the axon hillock to the terminus.
- The slight hyperpolarization induced by the opening of voltage-gated K+ channels also prevents the opening of Na+ channels upstream of an action potential.
 |
| Propagation of action potential |
1. Myelination and conduction velocity.
2. Flow of information depends on the rate at which action potentials are conducted in the nervous system.
3.The conduction of the action potential requires both active and passive flow of current
- Passive current: Diffusion of ions in the cell
- Active current: Through influx of Na+ ions
5. Alternative strategy is to insulate the axonal membrane
- Prevents current leakage and increases the distance along the axonal membrane that a current can flow passively
- Myelination of axons.
- Myelin sheath consists of multiple layers of closely opposed glial membranes wrapped around the axon.
- Oligodendrocytes in CNS and Schwann cells in PNS.
- Gaps present in myelin sheath known as Nodes of Ranvier.
- Axonal membrane comes in contact with the extracellular fluid only at the nodes.
- Na+/K+ pumps and voltage-gated Na+ channels located in the nodes.
- Action potential generation occurs only at these nodes.
- Action potential generated at one node elicits current that flows passively along the axon till the next node is reached.
- An action potential is generated at this node and the whole cycle is repeated.
- This process continues along the length of the axon.
- This type of propagation of signal conduction, where the action potential jumps from node to node is termed saltatory conduction.
 |
| Saltatory conduction of action potential along a myelinated axon |
Why are the transporters and channels
clustered in these regions?
1. Interaction of these proteins with
ankyrin and spectrin (cytoskeletal proteins)
2. Interaction of the extracellular
domain of the β1 subunit of the Na+ channel with the extracellular
domain of Nr-CAM (an adhesive protein
that is localized to the node)
3. Due to these protein-protein
interactions, the concentration of Na+ channels is higher at the
nodes.
4. Secretion of protein hormones by
glial cells that cause the clustering
5. of these nerve membrane proteins at
the nodes, by an unknown mechanism.
6. Presence of tight junctions between
the axon and the glial cell plasma membrane (glial cell is wrapped around the
axon) in the paranodal junctions immediately adjacent to the nodes; this may
prevent diffusion of proteins of Na+ channels and Na+/K+
pumps away from the nodes.
STRUCTURE OF VOLTAGE-GATED ION CHANNELS
1. Ion channels have some common
features
2. All are transmembrane proteins
3. They have a mechanism for sensing
changes in membrane potentials and responding to those changes appropriately,
i.e., by opening or closing the channels.
 |
| ION CHANNELS |
Voltage-gated K+ channels
1. Homotetrameric, with four subunits
arranged around a central pore, each containing 600-700 amino acids
2. Each subunit has six
membrane-spanning α-helices , S1-S6 and a P segment
- S5, S6 helices and P segment partially penetrate the membrane bilayer.
- Eight transmembrane helices (two from each subunit) form an inverted cone, forming the vestibule, a water-filled cavity in the central portion of the channel.
- Extended loops from the P segment form the ion selectivity filter.
- Present in the narrow part of the pore, near the exoplasmic surface.
- S4 helix – voltage sensor; contains positively charged amino acid residues Lys and Arg.
- S1 helix has the N-terminal channel-inactivating segment.
- Each monomeric subunit has one channel-inactivating segment.
 |
| VOLTAGE GATED K+ CHANNELS |
Voltage-gated Na+ channels
1. Monomeric
2. Four domains, I-IV
3. Domain structure similar to that of the
monomeric subunit of K+ channel
4. One major difference: Only one channel-inactivating
segment present in the protein.
 |
| VOLTAGE GATED Na+ CHANNELS |
NOTE: Ca+ channels also
have a similar structure.
SYNAPTIC TRANSMISSION:
Two types of synapses:-
1. Electrical synapses
2.Chemical synapses
Electrical synapses
- Passive flow of ionic current through gap junction between the presynaptic and postsynaptic neuron.
- Precisely aligned, paired channels present in the membranes of the two neurons at the synapse.
- Ions can diffuse through the gap junction pore, resulting in a passive flow of electrical current (which is initially generated by the action potential).
- Fast transmission, almost instantaneous.
- Synchronization of electrical
activity among populations of neurons.
o Brain stem neurons that generate rhythmic electrical activity underlying breathing are synchronized.§Populations of inter neurons in the cerebral cortex, thalamus, cerebellum, and other brain regions.o Some hormone-secreting neurons within the mammalian hypothalamus.
- Gap junction pores large enough to
allow ATP and second messengers to diffuse intercellularly
Permits electrical synapses to coordinate the
intracellular signaling and metabolism of coupled cells.
 |
| ELECTRICAL SYNAPSE |
Chemical synapses
1.Signaling mediated by chemicals,
i.e., neurotransmitters
2. Contained in synaptic vesicles in the
presynaptic terminal
3. Neurotransmitters secreted in the
synaptic cleft between the two neurons. The events triggering neurotransmitter
secretion are outlined below
o
Arrival
of an action potential at the axon terminal of the presynaptic neuron.
o
Opening
of voltage-gated Ca2+ channels in the presynaptic membrane and
influx of Ca2+
o
Fusion
of synaptic vesicles with the plasma membrane of the presynaptic neuron.
4. Bind to specific receptors on the
postsynaptic neuron.
5. Causes an alteration in the membrane
potential of the postsynaptic neuron.
NEUROTRANSMITTERS
1. Three criteria to be fulfilled for a
molecule to be considered a neurotransmitter.
2. The substance must be present within
the presynaptic neuron.
3. The substance must be released in response
to presynaptic depolarization, and the release must be Ca2+-dependent.
4. Specific receptors for the substance
must be present on the post-synaptic cell.
Neurotransmitter categories
Neurotransmitters categorized into:
- Small-molecule neurotransmitters
- Generally mediate rapid synaptic actions
- Peptide neurotransmitters
- Slower, ongoing synaptic functions
Some neurons synthesize and release
more than one type of neurotransmitter
- When more than one transmitter is present within a nerve terminal, the molecules are called co-transmitters.
- Differentially released depending on pattern of synaptic activity.
- Low frequency activity – Small neurotransmitters.
- High frequency activity -- Neuropeptides.
Synthesis and transport
- Small-molecule neurotransmitters.
- Synthesis occurs locally within presynaptic terminals.
- Enzymes required for synthesis produced in neuronal cell body and transported to the nerve terminal cytoplasm at 0.5–5 mm/day a day by slow axonal transport.
- Precursors taken into the nerve terminal by transporters present in the plasma membrane of the nerve terminal.
- Synthesized neurotransmitters loaded into synaptic vesicles.
- These vesicles are generally 40 to 60 nm in diameter, with their centers appearing clear in electron micrograph.
- These vesicles are referred to as small clearcore vesicles .
Neuropeptides
- Synthesized in the cell body of a neuron its site of secretion.
- Peptide-filled vesicles transported along an axon and down to the synaptic terminal via fast axonal transport.
- Transport at rates up to 400mm/day along microtubules by ATP-requiring “motor” proteins like kinesin.
- Packaged into synaptic vesicles 90 to 250 nm in diameter, which are electron-dense in electron micrographs; they are called large dense-core vesicles.
Removal of neurotransmitters: Carried
out by various mechanisms
- Diffusion away from the postsynaptic neuron in combination with reuptake into the presynaptic neuron or surrounding glial cells by specific transporters.
- Degradation of neurotransmitter by specific enzymes.
SYNAPTIC VESICLE CYCLE
Organization of the presynaptic
terminal
1. Secretion occurs on docking of
synaptic vesicles to specific active zones
2. Multiple pools of synaptic vesicles
present
- Ready releasable pool – undergo exocytosis in response to a single action potential as they have been primed by docking at the active zone.
- Recycled synaptic vesicle pool.
- Reserve pool – ensures that neurotransmitter is available even for the highest physiological demands.
3. Synaptic vesicles dock to a special
region of the plasma membrane at the synapse, the active zone.
4. Once docked, synaptic vesicle
proteins and plasma membrane proteins (v- and t-SNAREs) are closely apposed but
not activated.
5. The vesicles undergo a priming
process after docking and prior to
fusion
- Priming is ATP-dependent.
- Requires the activity of NSF (NEM-sensitive factor) and phosphoinositide transfer proteins.
- Priming prepares synaptic vesicles for fusing their membranes with the synaptic plasma membrane within milliseconds after an action potential arrives at the synapse.
- The proteins Munc13 and RIM (Rab3a-interacting molecule), localized to the active zone, are important in the priming process.
6. When an action potential reaches the
synapse, voltage-gated Ca2+ channels open resulting in an influx of Ca2+
ions flow into the synaptic terminal.
7. This raises the local intracellular
Ca2+ concentration in the vicinity of the active zone and docked vesicles.
8. Ca2+ ions bind Ca2+-binding
proteins, including synaptotagmin.
9. These proteins initiate vesicular membrane
fusion with the plasma membrane through a conformational change in both vesicle
and plasma membrane proteins.
 |
Synaptic vesicle
cycle
|
The synaptic vesicle cycle at the
molecular level
1. The ATPase NSF (NEM-sensitive fusion protein) and SNAPs (soluble NSF-attachment
proteins), play a role in priming synaptic vesicles for fusion.
2. Involved in regulating the assembly
of other proteins called SNAREs (SNAP
receptors).
- Synaptobrevin, SNARE protein found in the membrane of synaptic vesicles.
- Syntaxin and SNAP-25, SNARE proteins found primarily on the plasma membrane.
- These SNARE proteins can form a macromolecular complex that spans the two membranes, bringing them into close apposition.
- Toxins that cleave the SNARE proteins block neurotransmitter release
3. Many other proteins, such as complexin,
nSec-1, snapin, syntaphilin, and tomosyn, bind to the SNAREs and are probably
involved in the formation or disassembly of this complex.
4. Synaptotagmin, a protein found in the
membrane of synaptic vesicles, is possibly involved in Ca2+
regulation of neurotransmitter release
5. Synaptotagmin binds Ca2+
at concentrations similar to those required to trigger vesicle fusion within
the presynaptic terminal.
6. Probably acts as a Ca2+
sensor, signaling the elevation of Ca2+ ion concentration within the terminal
and thereby triggering vesicle fusion.
- Evidence to support this hypothesis.
- Alterations of the properties of synaptotagmin in the presynaptic terminals of mice, fruit flies, squid, and other experimental animals impair Ca2+-dependent neurotransmitter release.
- Deletion of only one of the 19 synaptotagmin genes of mice is a lethal mutation, causing the mice to die soon after birth.
7. Model for synaptotagmin role in exocytosis
- Binding of Ca2+ is known to change the chemical properties of synaptotagmin, allowing it to insert into membranes and to bind to other proteins, including the SNAREs.
- It is possible that the SNARE proteins bring the two membranes close together, and that Ca2+-induced changes in synaptotagmin then produce the final fusion of these membranes.
8. Other proteins appear to be involved
at subsequent steps of the synaptic vesicle cycle
9. Clathrin is involved in endocytotic
budding of vesicles from the plasma membrane.
- Clathrin forms structures which are involved in formation of coated pits that initiate membrane budding.
- Accessory proteins, such as AP2, AP180 and amphiphysin are involved in assembly of individual clathrin triskelia into coats.
- The coats increase the curvature of the budding membrane until it forms a coated vesicle-like structure.
- The protein dynamin, is involved in the final pinching-off of membrane to convert the coated pits into coated vesicles.
- The coats are then removed by an ATPase, Hsc70, with another protein called auxilin serving as a co-factor.
- Other proteins, like synaptojanin, are also involved in vesicle uncoating.
10. The protein synapsin, which
reversibly binds to synaptic vesicles, may cross-link newly formed vesicles to
the cytoskeleton to keep the vesicles tethered within the reserve pool.
11. Mobilization of these reserve pool
vesicles is caused by phosphorylation of synapsin by proteins kinases.
12. The phosphorylated synapsin dissociates
from the vesicles, thus freeing the vesicles to make their way to the plasma
membrane.
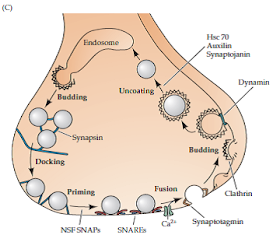 |
| ACTION OF SNAREs |
NEUROTRANSMITTER
Acetylcholine
1. First substance to be identified as a
neurotransmitter
2. Functions as a neurotransmitter at
o
Skeletal
neuromuscular junctions
o
Neuromuscular
synapse between the vagus nerve and cardiac muscle fibers
o
Synapses
in the ganglia of the visceral motor system
o
Variety
of sites within the central nervous system
Receptors
3. Nicotinic acetylcholine receptor –
nAChR
o
Nicotine
is an agonist
o
Ionotropic
receptor
4. nAChR in skeletal muscle:
o
Pentameric
protein with composition α2βγδ.
o
Cation
channel is a tapered central pore lined by homologous segments from each of the
five subunits
o
Receptor
cooperatively binds two acetylcholine molecules to sites located at the
interfaces of the αδ and αγ
subunits.
o
Channel
is opened within a few microseconds.
o
Open
ion channel is, at its narrowest, about 0.65–0.80 nm in diameter.
o
Sufficient
to allow passage of both Na+ and K+ ions with their shell
of bound water molecules.
o
Acetylcholine
receptor probably transports hydrated ions, unlike Na+ and K+
channels,
o
Subunit
structure
§ Each subunit has four transmembrane
domains
§ Constitute the ion channel portion of
the receptor
§ Long extracellular region constitutes the ACh-binding domain
o
Ion
channel
§ Lined by five homologous
transmembrane M2 α helices, one from
each of the five subunits.
§ Two acetylcholine binding sites in
the extracellular domain of the receptor lie ≈4 to 5 nm from the center of the
pore
§ Five M2 helices rotate relative to
the vertical axis of the channel during opening and closing
§ Helices composed largely of
hydrophobic or uncharged polar amino acids
§ Negatively charged aspartate or
glutamate residues located at each end, near the membrane faces
§ Several serine or threonine residues
are near the middle.
§ Aspartate and glutamate residues help
to screen out anions and attract Na+ or K+ ions as they
enter the channel
§ Similar ring of negative charges
lining the cytosolic pore surface also helps select cations for passage
§ Mutation studies frog oocytes
§ Single negatively charged glutamate
or aspartate in one M2 helix is replaced by a positively charged lysine
§ The number of ions that pass through
during the open state is reduced.
§ Greater the number of acidic residues
mutated, more the decrease in conductivity.
 |
| ACETYL CHOLINE RECEPTOR |
5. Muscuranic acetylcholine receptor –
mAChR
o
Present
in cardiac muscle
o
Metabotropic
receptor
o
Inhibitory
o
Slow
the rate of heart muscle contraction by causing a sustained hyperpolarization.
o
GPCR.
Glutamate
1. Most important neurotransmitter in
normal brain function
2. Almost all excitatory neurons in the CNS are glutamatergic; estimated that over half of
all brain synapses release this agent
3. Receptors, both ionotropic and
metabotropic
4. Ionotropic
o
Ionotropic
glutamate receptors named according to the agonists that they bind
§ NMDA receptors (N-methyl-D-aspartate)
§ AMPA receptors (α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate)
§ Kainate receptors
o
Nonselective
cation channels, allow passage of Na+ and K+
o
Different
subtypes of receptor composed of 4-5 subunits
o
Unique
properties of NMDA receptors
§ Allow the passage of Ca2+
in addition to Na+ and K+
·
Ca2+
concentration can be increased in the postsynaptic neuron; Ca2+ can
act as a second messenger and activate intracellular signaling cascades
§ Bind extracellular Mg2+
·
Mg2+
blocks the receptor channel when membrane is hyperpolarized
·
Depolarization
pushes Mg2+ out of the channels, allowing other cations to flow
§ Opening of the NMDA receptor channel
requires glycine as a co-agonist
o
Most
glutamatergic synapses have both AMPA and NMDA receptors, only a few have only
one type (AMPA or NMDA).
o
Metabotropic
glutamate receptors(mGluR)
§ Linked by trimeric G proteins to
cytoplasmic enzymes.
§ Eight mGluRs(mGluR1-R8) cloned so far.
§ Grouped into 3 functional classes
based on based on amino-acid sequence homology, agonist pharmacology and the
signal transduction pathways to which they are coupled.
§ Group I mGluRs stimulate
phospholipase C activity and the release of Ca2+ from cytoplasmic stores.
§ Activation of Group II and III mGluRs causes inhibition of adenylate cyclase.
§ Activation of mGluR closes
voltage-dependent, Ca2+-dependent K+ channels in hippocampal and other cortical
neurons.
§ Cause slower postsynaptic responses which
can either increase or decrease the excitability of postsynaptic cells.
 |
| GLUTAMATE RECEPTOR |
- γ-Aminobutyric acid
1. Inhibitory neurotransmitter2. Almost a third of the synapses in the brain use GABA3. Local circuit interneurons4. Three types of receptorso GABAA, GABAC ionotropic§ Ionotropic GABA receptors inhibitory Cl- channels§ Pentameric (α,β,γ,δ,ρ)o GABAB metabotropic§ Metabotropic GABA receptors also inhibitory, activate K+ channels§ Another mode of action involves the blocking of Ca2+ channels, which causes hyperpolarization of postsynaptic cell§ Heterodimers of GABAB R1 and R2 subunits.
 |
| Ionotropic GABA receptor |
- Glycine
1. Inhibitory neurotransmitter.2. Ligand-gated Cl– channels.3. Pentamers, α4β, α subunits involved in glycine-binding.
- Catecholamines
1. Dopamine, epinephrine, norepinephrine2. Dopamineo Major dopamine-containing area of the brain is the corpus striatum, which receives inputs from the substantia nigra, and is involved in coordination of body movementso In Parkinson’s disease, degeneration of the dopaminergic neurons of the substantia nigra, leads to the characteristic motor dysfunction.o Also thought to be involved in motivation, reward, and reinforcemento Dopamine receptors are G protein-coupled receptors§ Five receptor subtypes , D1-D5§ D1 and D5(D1-like) ; D2, D3, D4 (D2-like)§ D1-like activate adenylyl cyclase while D2-like inhibit adenylyl cyclase3. Epinephrine and norepinephrine receptors (adrenergic receptors)o G protein-coupled receptorso Nine distinct receptor subtypes, classified into three subfamilies, α1,α2 and βo α1-adrenergic receptor subtypes activate Gq/11, increasing PLC β activity (releasing IP3 and DAG).o α2-adrenergic receptor subtypes all activate Gi resulting in inhibition of adenylyl cyclase activity.β-adrenergic receptor subtypes all activate GS to increase adenylyl cyclase activity.
- Serotonin
1. Three families of serotonin receptors
2. All except for 5-HT3,
which is a ligand-gated ion channel, are
GPCRs
3. 5-HT1 receptor family
comprises the 5-HT1A,5-HT1B, 5-HT1D, 5-ht1E
and 5-ht1F receptors; inhibition of adenylyl cyclase.
4. 5-HT2 receptor family
comprises the 5-HT2A, 5-HT2B and 5HT2C receptors;
activate the Gq/11 family of
G proteins (phospholipase C signaling cascade).
5. 5-HT3 receptor is a ligand-gated
ion channel; pentameric; subunit composition not known.
6. 5-HT4, 5-ht6
and 5-HT7 receptors are activate the Gs family of G
proteins and thus cause the stimulation of adenylyl cyclase.
7. 5-ht5 receptor is an
orphan receptor.
- Histamine receptor
1. Histamine found in neurons of the hypothalamus that send sparse but widespread projections to almost all regions of the brain and spinal cord2. The central histamine projections mediate arousal and attention,3. Four types of histamine receptors, all GPCRso H1 linked to Gq family of G proteins; it can also stimulate phospholipase A2 activity, with the release of arachidonic acid§ Can also stimulate cGMP synthesis in brain§ Outside the brain, can cause relaxation of vascular smooth muscle via synthesis and release of nitric oxide.o H2 receptors activate the Gs family of G proteins and stimulate cAMP synthesis; could possibly activate Gq family of G proteins.o H3 receptors are linked to Gi/o type of G proteins.H4 receptors are orphan receptors, no information on signaling in the CNS.
- PEPTIDE NEUROTRANSMITTERS
1. Many peptides which act as hormones also function as neurotransmitters2. Biological activity depends on their amino acid sequence3. Classified into 5 categories, depending on amino acid sequenceo Brain/gut peptides. Eg. Substance P, vasoactive intestinal peptide(VIP), cholecystokinin octapeptide (CCK-8)o Opioid peptides. Eg. Leucine enkephalin, α-Endorphin, Dynorphin Ao Pituitary peptides. Eg. Vasopressin, oxytocin, ACTHo Hypothalamic-releasing hormones. Eg. Thyrotropin releasingo hormone (TRH), Leutinizing hormone-releasing hormone (LHRH), Somatostatin-1o Miscellaneous peptides. This category contains other peptides that are not easily classified. Eg. Angiotensin-II, neuropeptide-γ, neurotensin4. The synthesis of peptide neurotransmitters involves processing of a larger polypeptide precursor5. Prepropeptides synthesized in RER6. Cleavage of the signal sequence yields propeptides which are larger than the mature peptide neurotransmitter7. They are further processed and modified to yield the mature peptide8. Processing involves proteolytic cleavage, modification of the ends of the peptide, glycosylation, phosphorylation, and disulfide bond formation.9. Peptides are catabolized into inactive amino acid fragments by peptidases, usually located on the extracellular surface of the plasma membrane.Neuropeptides generally activate GPCRs.
Opioid Peptides
1. Opioid peptides, so called because
they bind to the same postsynaptic receptor activated by opium; active
ingredients in opium are a variety of plant alkaloids, predominantly morphine
2. Discovered during the 1970s during a
search for endogenous compounds mimicking the action of morphine,
or endorphins
3. Opioid peptides categorized into 3
classes,
o
Endorphins(α-Endorphin,
α-Neoendorphin, β-Endorphin, γ-Endorphin)
o
Enkephalins (Leu-enkephalin,Met-enkephalin)
o
Dynorphins
(Dynorphin A, Dynorphin B)
o
Opioid
peptides widely distributed throughout the brain; often co-localized with other
small-molecule neurotransmitters, like GABA and 5-HT.
o
Tend
to act as depressants
o
Act
as analgesics on intracerebral injection in experimental animals
o
Are
also involved in complex behaviors like sexual attraction and
aggressive/submissive behaviors
4. Opioid receptors
o
GPCRs
o
Three
receptor subtypes μ, κ and δ
o
μ
receptor: β-endorphin and endomorphins-1 and -2
o
δ
receptors : enkephalins
o
κ
receptors: dynorphins
5. Enkephalins
o
Pentapeptides
involved in nociception (pain perception)
o
Leu-enkephalin
(Tyr-Gly-Gly-Phe-Leu)
o
Met-enkephalin (Tyr-Gly-Gly-Phe-Met)
o
Precursor
pre-proenkephalin encodes multiple copies of Met-enkephalin, including two extended
forms of Met-enkephalin (a heptapeptide and an octapeptide) and a single copy
of Leu-enkephalin
 |
| GENE FOR ENKEPHALIN |
Enkephalin synthesis
Enkephalins bind to δ subtype of
opioid receptors
Somatostatin
o
Two
forms which are 14 and 28 residues long
o
neurotransmitter/hormone
with several biologial functions
o
Neuroendocrine
system
§ Iinhibits secretion of growth hormone
and prolactin in the anterior pituitary
§ Inhibits secretion in the intestine
(including gastric acid in the stomach), pancreatic acinar cells and pancreatic
beta-cells
§ Stimulates absorption in the intestine and
modulates smooth muscle contractility.
o
In
the CNS, acts as a neurotransmitter
§ Hyperpolarizes cell membranes by opening K+
channels
§ Also selectively inhibit high
voltage-activated Ca2+ channels in hippocampal pyramidal neurons
§ Probably involved in important role
in regulating locomotor activity and cognitive function
§ Receptors- GPCRs
§ Five receptor subtypes
§ Inhibit adenylyl cyclase.



Deletion of only one of the 19 synaptotagmin genes of mice is a lethal mutation, causing the mice to die soon after birth.—crispr mice
ReplyDeleteDynorphin A(1-13), a version of dynorphin A containing only the first 13 amino acids of the peptide, in the rat spinal cord has additive effects. When dynorphin A1-13 was injected into the intracerebroventriulcar (ICV) region of the brain, Dynorphin A(1-13)
ReplyDelete