In 1958, Francis Crick postulated the existence of an adaptor molecule, presumably RNA, that could serve as a mediator between the string of nucleotides in DNA (actually in mRNA) and the string of amino acids in the corresponding protein. Crick favored the idea that the adapter contained two or three nucleotides that could pair with nucleotides in codons, although no one knew the nature of codons, or even of the existence of mRNA, at that time. Transfer RNA had already been discovered by Paul Zamecnik and coworkers a year earlier, although they did not realize that it played an adapter role.
The Discovery of tRNA
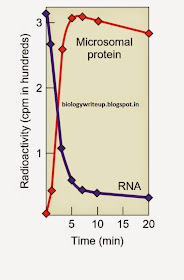
By 1957, Zamecnik and colleagues had worked out a cell free protein synthesis system from the rat. One of the components of the system was a so-called pH 5 enzyme fraction that contained the soluble factors that worked with ribosomes to direct translation of added mRNAs. Most of the components in the pH 5 enzyme fraction were proteins, but Zamecnik’s group discovered that this mixture also included a small RNA. Of even more interest was their finding that this RNA could be coupled to amino acids. To demonstrate this, they mixed the RNA with the pH 5 enzymes, ATP, and [14C] leucine. Figure 19.26a shows that the more labeled leucine these workers added to the mixture, the more was attached to the RNA, which they separated from protein by phenol extraction. Furthermore, when they left out ATP, no reaction occurred. We now know that this reaction was the charging of tRNA with an amino acid. Not only did Zamecnik and his coworkers show that the small RNA could be charged with an amino acid, they also demonstrated that it could pass its amino acid to a growing protein. They performed this experiment by mixing the [14C]leucine-charged pH 5 RNA with microsomes— small sections of endoplasmic reticulum containing ribosomes. Figure 19.26b shows a near-perfect correspondence between the loss of radioactive leucine from the pH 5 RNA and gain of the leucine by the protein in the microsomes. This represented the incorporation of leucine from leucyltRNA into nascent polypeptides on ribosomes.


great pleasure reading your post.Its full of information I looking for and I love to post a comment that "The content of your post is awesome" Great work.
ReplyDeleteGastroenterologist
Your website is really cool and this is a great inspiring article.
ReplyDeletehttps://dynamichealthstaff.com/nursing-jobs-in-uk-for-indian-nurses
This comment has been removed by the author.
ReplyDeleteVisit my blog
ReplyDelete